|
|
The Brain Simulation Platform "Live Papers" |
|
|
The Brain Simulation Platform "Live Papers" |
Authors: Lupascu CA1, Morabito A2, Ruggeri F2, Parisi C2, Pimpinella D2, Pizzarelli R2, Meli G2, Marinelli S2, Cherubini E2, Cattaneo A2, and Migliore M1
Author information: 1Institute of Biophysics, National Research Council, Palermo, Italy, 2 European Brain Research Institute, Rome, Italy.
Corresponding author: Author (Lupascu CA carmen.lupascu@pa.ibf.cnr.it )
Journal: Frontiers in Cellular Neuroscience
Download Url: https://www.frontiersin.org/articles/10.3389/fncel.2020.00173/abstract
Citation: Lupascu CA, Morabito A, Ruggeri F, Parisi C, Pimpinella D, Pizzarelli R, Meli G, Marinelli S, Cherubini E, Cattaneo A & Migliore M (2020). Computational modeling of inhibitory transsynaptic signaling in hippocampal and cortical neurons expressing intrabodies against gephyrin. Frontiers in Celllular Neuroscience, In press.
Licence: the Creative Commons Attribution (CC BY) license applies for all files. Under this Open Access license anyone may copy, distribute, or reuse the files as long as the authors and the original source are properly cited.A reduced self-consistent set of files needed to reproduce the fittings in the paper is available on ModelDB. The kinetic model of synaptic transmission used in the paper is schematically illustrated below (Figure 1 of the paper):
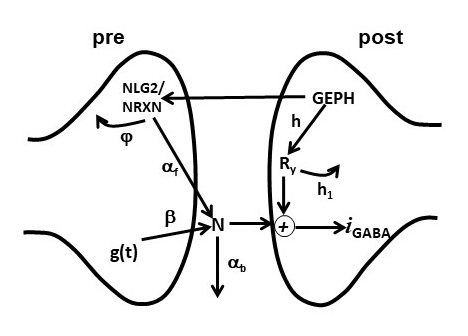 We modeled the action of the variables GEPH(gephyrin clusters), NLG2 (Neuroligin/Neurexin clusters), N (Neurotransmitter molecules), and Ry (Postsynaptic receptors) through the following equations:
$$\frac{dN}{dt} = \beta \cdot \alpha_{f} \cdot g(t) \cdot NLG2 - \alpha_{b} \cdot N$$
$$\frac{dNLG2}{dt} = \frac{GEPH}{1+\frac{GEPH}{2 \cdot NLG2}}-\phi \cdot NLG2$$
$$\frac{dR_{y}}{dt} = h \cdot GEPH - h_{1} \cdot R_{y}$$
after a synaptic activation, g(t) generates a number of neurotransmitter molecules, N, at a rate \(β\).
The synaptic current was calculated as:
$$I_{GABAA} = c_{1} \cdot N \cdot R_{y} \cdot (v-e_{rev})$$ where \(c_{1}\) is a constant, v the membrane potential and \(e_{rev}\) the reversal potential.
The set of differential equation can be solved analytically. The current \(I_{GABAA}\) can be described as:
$$I_{GABAA} = I_{FACT} \cdot \frac{[(1-\alpha_{b}\tau_{d})-(1-\alpha_{b}\tau_{r})] \cdot e^{-\alpha_{b}\cdot t}+(1-\alpha_{b}\tau_{r}) \cdot e^{\frac{-t}{\tau_d}}-(1-\alpha_{b}\tau_{d}) \cdot e^{\frac{-t}{\tau_{r}}}}{(1-\alpha_{b}\tau_{d})(1-\alpha_{b}\tau_{r})} \cdot (v-e_{GABAA})$$
where $$I_{FACT} = c_{1} \cdot \frac{h}{h_{1}} \cdot [\frac{(2-\phi)\cdot GEPH^{2}}{2\cdot\phi}] \cdot \beta \cdot \alpha_{f} \cdot w$$
We modeled the action of the variables GEPH(gephyrin clusters), NLG2 (Neuroligin/Neurexin clusters), N (Neurotransmitter molecules), and Ry (Postsynaptic receptors) through the following equations:
$$\frac{dN}{dt} = \beta \cdot \alpha_{f} \cdot g(t) \cdot NLG2 - \alpha_{b} \cdot N$$
$$\frac{dNLG2}{dt} = \frac{GEPH}{1+\frac{GEPH}{2 \cdot NLG2}}-\phi \cdot NLG2$$
$$\frac{dR_{y}}{dt} = h \cdot GEPH - h_{1} \cdot R_{y}$$
after a synaptic activation, g(t) generates a number of neurotransmitter molecules, N, at a rate \(β\).
The synaptic current was calculated as:
$$I_{GABAA} = c_{1} \cdot N \cdot R_{y} \cdot (v-e_{rev})$$ where \(c_{1}\) is a constant, v the membrane potential and \(e_{rev}\) the reversal potential.
The set of differential equation can be solved analytically. The current \(I_{GABAA}\) can be described as:
$$I_{GABAA} = I_{FACT} \cdot \frac{[(1-\alpha_{b}\tau_{d})-(1-\alpha_{b}\tau_{r})] \cdot e^{-\alpha_{b}\cdot t}+(1-\alpha_{b}\tau_{r}) \cdot e^{\frac{-t}{\tau_d}}-(1-\alpha_{b}\tau_{d}) \cdot e^{\frac{-t}{\tau_{r}}}}{(1-\alpha_{b}\tau_{d})(1-\alpha_{b}\tau_{r})} \cdot (v-e_{GABAA})$$
where $$I_{FACT} = c_{1} \cdot \frac{h}{h_{1}} \cdot [\frac{(2-\phi)\cdot GEPH^{2}}{2\cdot\phi}] \cdot \beta \cdot \alpha_{f} \cdot w$$
|
|
|||
| \(h\) |
|
\(τ_{r}\) |
|
| \(h_{1}\) |
|
\(φ\) |
|
| \(α_{f}\) |
|
\(GEPH\) |
|
| \(α_{b}\) |
|
\(w\) |
|
| \(β\) |
|
\(v\) |
|
| \(τ_{d}\) |
|
\(e\) |
|
|
|
|
||
|
|
|||